The key laboratory for Veterinary Biotechnology meet 94 criteria of ISO/IEC 17025:2017
Updated on 08:02, Thursday, 22/04/2021 (GMT+7)
In most countries, ISO/IEC 17025 is the standard that most laboratories must apply and satisfy to be considered technically competent. In many cases, service users, managing authorities or third parties will not accept the trial test or calibration results from a laboratory that does not meet the ISO/IEC17025 standard. The latest version of ISO/IEC17025 criteria was issued in 2017 (since 1990, ISO/IEC has had 4 versions issued in 1990, 1999, 2005 and 2017).
In most countries, ISO/IEC 17025 is the standard that most laboratories must apply and satisfy to be considered technically competent. In many cases, service users, managing authorities or third parties will not accept the trial test or calibration results from a laboratory that does not meet the ISO/IEC17025 standard. The latest version of ISO/IEC17025 criteria was issued in 2017 (since 1990, ISO/IEC has had 4 versions issued in 1990, 1999, 2005 and 2017).
According to the ISO regulations, the ISO/IEC 17015:2005 version expired in 2020. Therefore, the key laboratory on Veterinary Biotechnology, Faculty of Veterinary Medicine, VNUA has assessed 94 experiment criteria according to ISO/IEC 17025:2017 standards under the supervision of "Accreditation Office For Standards Conformity Assessment Capacity-AOSC”. Then, the key laboratory on Veterinary Biotechnology has been granted the ISO/IEC 17025:2017 Certification for 94 criteria of disease testing on land animals and aquatic animals (Certificate image and list of ISO criteria). Certificates are valid for 5 years from the date of issue. In the future, the Laboratory will continue to maintain and develop more experiment criteria according to ISO/IEC 17025:2017 standards to meet the requirement of research as well as production practice.
Some pictures of AOSC assessment team - the key laboratory on Veterinary Biotechnology

The launching meeting of the assessment team

Picture of the evaluation team and laboratory team
Some pictures from the laboratory
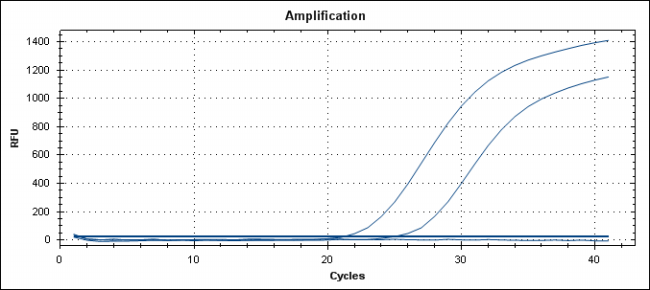
The illustration image of the skill assessment for the criteria "Detecting Newcastle virus by Realtime RT-PCR technique"
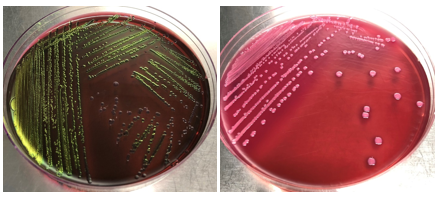
The illustration image of the skill assessment for the criteria "Isolation of E. coli bacteria"
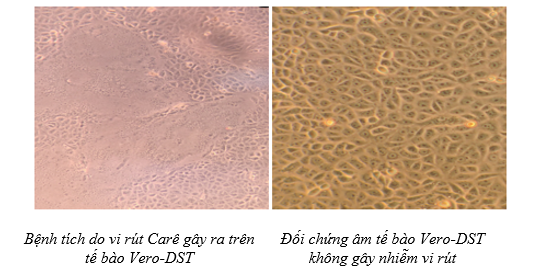
The illustration image of the skill assessment for the criteria "Isolation of the Carea Virus"
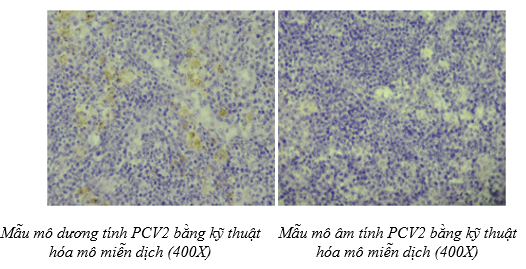
The illustration image of the skill assessment for the criteria "Detecting PCV2 by immunohistochemistry technique"
By Faculty of Veterinary Medicine